The doc discusses GMP compliance audits. It defines GMP audits to be a approach to verify that brands comply with great production procedures polices. There are 2 types of audits - onsite audits, which contain traveling to the production web site, and desktop audits, which overview documentation without a web site check out.
QUALIFICATION & VALIDATION.Validation is An important Component of GMP, and an element of QA.Crucial actions in the method need to be validated.Need to have for self esteem the products will constantly meet predetermined specifications and characteristics.
Are you aware a perfectly-executed Quality Assurance Audit can conserve your business thousands & Strengthen customer have faith in? Discover the shocking strategies that major companies use to ace their audits! ✅
The documentation segment of your checklist handles all elements of documentation connected to the manufacturing method. This includes a overview on the strategies for doc Manage, such as the issuance, retrieval, and archiving of files.
This doc discusses cleansing validation, which offers documented evidence that accepted cleaning methods will create devices appropriate for processing pharmaceutical items. It defines different levels of cleaning validation based upon possibility.
Audit trail is nowadays integral Section of pharmaceutical industry. If audit trail is not done some key effect is usually noticed on industry which include;
two. Filter leak tests and particulate counting to examine filter performance and air quality. 3. Pressure differential, temperature, humidity, and sound stage tests to validate environmental controls. Validation with the HVAC procedure is important to display that it could continuously source air Assembly top quality benchmarks to maintain aseptic manufacturing problems.
” FDA recommends that audit trail that seize changes to essential data be reviewed with record and just before last approval of the report. Audit trail issue to standard assessment consists of, but usually are not restricted to; the modify heritage of completed item test outcomes, changes to simple run sequences, variations to sample identification, and modifications to critical process parameters.
The doc discusses distinct types of audits done check here during the pharmaceutical industry. It defines internal audits as self-audits done in just a company to be certain compliance and identify places for improvement.
With sturdy internal treatments, our excellent method and our auditor qualification procedure, pushed by our Top quality supervisor, is consistently audited by our clientele with optimistic results of reliability and robustness.
Info Integrity Audits: Discover the rising importance of knowledge integrity audits in pharmaceutical companies to ensure the accuracy and reliability of knowledge used in regulatory submissions.
This really helps to quickly and easily comprehend the issue with out confusion. Simple issue definition results in productive and accurate remedies, causing improved approach enhancement and high-quality.
Challenge definition or presentation needs to be uncomplicated, directed at standard personnel with simple course of action understanding.
Insufficient audit definition in pharmaceutical industry or inadequate documentation is a standard challenge throughout audits. Missing batch documents, incomplete SOPs, and lack of appropriate modify controls can result in compliance problems.
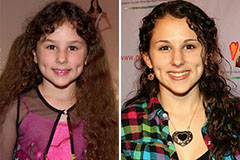 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Romeo Miller Then & Now!
Romeo Miller Then & Now! Destiny’s Child Then & Now!
Destiny’s Child Then & Now!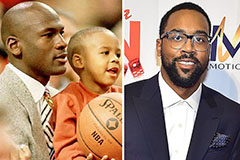 Marcus Jordan Then & Now!
Marcus Jordan Then & Now! Andrew McCarthy Then & Now!
Andrew McCarthy Then & Now!